For health professionals, birthing facilities, and local public health agencies (LPHAs)
For general reporting of all reportable conditions, including Hepatitis B, see our Report-a-Disease page. If reporting a Hepatitis B case, use the General Disease Reporting Form and fax it to 303-782-0338.
All positive test results (HBsAg, IgM anti-HBc, HBeAg, or HBV DNA) from laboratories and facilities must be reported to the Colorado Department of Public Health and Environment within four days, as required by the rules and regulations pertaining to epidemic and communicable disease control set forth by the Colorado Board of Health in 6 CCR 1009-1. Visit CDC’s webpage to review content regarding HIPAA requirements and perinatal hepatitis B prevention.
Once a report is received, the Perinatal Hepatitis B Prevention Program (PHPBB) Coordinator determines if the person who is pregnant or postnatal is within the parameters of the program. Eligible infants are then monitored until post-vaccination serologic testing (PVST) is completed, which assesses the child for infection or immunity and directs further care.
Birthing facilities should complete the HBIG form for every newborn receiving hepatitis B immune globulin (HBIG) within 24 hours of delivery.
Perinatal HepB forms:
| Report HBIG administration For reporting HBIG and the initial dose of HepB vaccine administration within 24 hours of delivery | Report HepB vaccine series and PVST results For reporting subsequent HepB vaccine administrations and post-vaccination serologic testing (PVST) | |
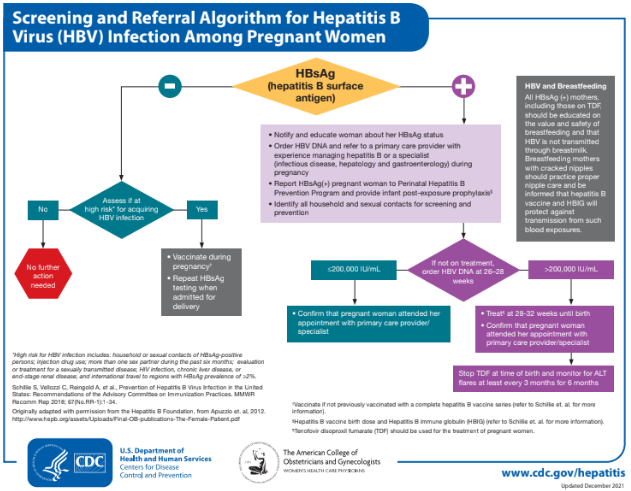
Per CDC, all people who are pregnant should be tested for HBsAg during an early prenatal visit (e.g., first trimester) in each pregnancy, even if they have been vaccinated or tested previously. Testing people who are pregnant and known to be chronically infected with HBV, provides documentation of the positive HBsAg test result obtained during pregnancy and helps to ensure that their infants will be identified for timely prophylaxis.
People who are pregnant and not tested prenatally, those with clinical hepatitis, and those at increased vulnerability to acquiring HBV (e.g., recent or current injection-drug use, having had more than one sex partner in the previous six months or an HBsAg-positive sex partner, having been evaluated or treated for an STI) should be tested at the time of admission to the hospital or birthing facility for delivery. High-risk individuals should be tested at admission even if they previously tested negative during routine prenatal screening.
Management of infants with known OR unknown* perinatal exposure of HepB virus (HBV)
*If a birthing person has not been screened prenatally or if test results are not available at the time of admission for delivery, HepB surface antigen (HBsAg) testing should be collected at the time of admission to a hospital or other delivery setting.
If the birthing person is HBsAg positive, the infant should receive hepatitis B immune globulin (HBIG) as soon as possible (within 12 hours of birth). HepB vaccine should be administered in a separate limb, within 12 hours of birth.
If the birthing person was not screened prenatally, the infant should receive HepB vaccine within 12 hours of birth. Infants weighing less than 2,000 grams should also receive HBIG within 12 hours of birth. The birthing person’s HBsAg status should be assessed as soon as possible. If the birthing person is determined to be HBsAg-positive, infants weighing at least 2,000 grams should also receive HBIG as soon as possible but no later than seven days after birth .
If evidence is suggestive of HepB infection (e.g., there is presence of HBV DNA, the birthing person is HBeAg-positive or is known to have chronic hepatitis B infection), or the birthing person’s status remains unknown, manage the infant as if the birthing person is HBsAg-positive.
|
|
|||||
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
||
|
|
|
|
|
|
||
†Administer the final dose no earlier than 6 months old (minimum age 24 weeks; includes 4-day grace period).
For more information, see chapter 10 (HepB) in the Epidemiology and Prevention of Vaccine-Preventable Diseases; MMWR: Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the ACIP, 2018.
Management of infants with no perinatal exposure of HepB virus (HBV)
Vaccinate children following recommendations from CDC.
- 3-dose series beginning at birth* (within 24 hours) and then at age 1–2 months and 6–18 months. (The use of combination vaccines are not permitted prior to 6 weeks old; use single-antigen HepB vaccine for infants less than 6 weeks old).
- Administration of 4 doses is permitted when a combination vaccine containing HepB is used after the birth dose (at 2, 4, and 6 months of age).
- Minimum age for the final (3rd or 4th) dose: 24 weeks.
- Infants who did not receive a birth dose should begin the series as soon as possible (see Table 2).
- *For infants weighing <2,000 grams, provide the birth dose at hospital discharge or 1 month old.
Post-vaccination serologic testing (PVST)
PVST is recommended for infants and children born to people with hepatitis B infection. Serologic testing confirms whether the child has developed immunity or has been infected with HBV.
- PVST should only include:
- HepB surface antigen (HBsAg).
- HepB surface antibody (anti-HBs).
PVST should occur between 9–12 months old or one to two months after vaccine series completion, if the series is delayed. Testing should not be performed before 9 months of age to avoid detection of passive anti-HBs from HBIG administered at birth and to maximize the likelihood of detecting late HBV infection. Antibodies to hepatitis B core antigen (anti-HBc) testing of infants is not recommended because passively acquired parental anti-HBc might be detected in infants born to HBsAg-positive birthing parents up to 24 months.
Note: Tests for antibodies to hepatitis B core antigen (anti-HBc) should not be performed.
Management of Infants Born to Women with Hepatitis B Virus Infection for Pediatricians (PDF)
Interpretation of PVST
Interpreting post-vaccination serologic test (PVST) - higher resolution version of the above image which also includes two more pages (PDF)
For more information, see chapter 10 (HepB) in the Epidemiology and Prevention of Vaccine-Preventable Diseases; MMWR: Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the ACIP, 2018.
HepB provider tip sheet: FAQs
Hepatitis B virus (HBV) is a pathogen which causes vaccine-preventable liver infection. Between 880,000 to 1.89 million people are living with HBV infection in the United States, two-thirds of whom may be unaware of their infection. The infection can be acute or chronic. Chronic infections can lead to cirrhosis, liver cancer, and premature death. Though usually asymptomatic, most infants (90%) who are infected with HBV will develop chronic infection and 25% will die prematurely from liver cancer or cirrhosis. HBV is transmitted through contact with infectious blood or body fluids, or from a person who is infected (HBsAg+) to their newborn during delivery.
Yes, perinatal transmission can be prevented by screening for HBsAg during every pregnancy. Infants born to HBsAg+ people should receive hepatitis B immune globulin (HBIG) and a dose of single-antigen hepatitis B vaccine within 12 hours of birth, followed by a complete series of the hepatitis B vaccine. This combination is up to 94% effective in preventing perinatal transmission.
The infant should receive an urgent referral to receive HBIG, which can be administered up to seven days after birth. If more than seven days have passed, HBIG is unlikely to be effective in preventing transmission. However, it is still important for the infant to complete the hepatitis B vaccine series, and providers should adhere to the minimum intervals between doses.
Post-vaccination serologic testing (PVST) is recommended for infants and children born to people with hepatitis B infection. Serologic testing confirms whether the child has developed immunity or if they have been infected with HBV. PVST should include hepatitis B surface antigen (HBsAg) and hepatitis B surface antibody (anti-HBs) only. PVST should occur between 9–12 months old or one to two months after vaccine series completion if the series is delayed. Testing should not be performed before 9 months to avoid detection of passive anti-HBs from HBIG administered at birth and to maximize the likelihood of detecting late HBV infection. Testing for antibodies to hepatitis B core antigen (anti-HBc) testing of infants is not recommended because passively-acquired parental anti-HBc might be detected in infants born to HBsAg-positive birthing parents up to 24 months.
In infants, a positive anti-HBc test may result from measuring antibodies passively acquired from the birthing parent, which may be detectable in HBV-exposed infants up to 24 months old.
Testing performed before 9 months can provide inaccurate anti-HBs results by detecting passive antibodies from HBIG administered at birth rather than actual response to the hepatitis B vaccine. Also, for infants who receive HBIG at birth, there can be a prolonged HBV incubation period. Waiting until 9 months old can maximize detection of late HBV infection, if present.
No, transient HBsAg positivity has been reported for up to 18 days after vaccination. To ensure accurate PVST results, the test must be conducted at 9–12 months old or one to two months after vaccine series completion if the series is delayed.
No, anti-HBs concentrations decline rapidly within the first year after the series is completed. Delaying PVST beyond the recommended timeframe may yield a negative/non-reactive anti-HBs result, making it difficult to determine if immunity has waned or if the infant did not respond to the vaccine. This ambiguity may lead to unnecessary re-vaccination. For this reason, providers are encouraged to test at 9–12 months old or one to two months after vaccine series completion if the series is delayed.
Yes, CDC provides funding and technical assistance for perinatal hepatitis B prevention programs (PHBPPs) in all 50 states and 14 other jurisdictions. All infants perinatally-exposed to HBV should be reported to the PHBPP. The Perinatal Hepatitis B Prevention Case Coordinator is Leovi Madera, BSN, RN.
Contact information:
leovi.madera@state.co.us
Cell 303-870-6263
Fax 303-759-5257
For Perinatal Hepatitis B questions, contact the Perinatal Hepatitis B Prevention Program (PHBPP) coordinator:
Leovi Madera, BSN, RN
Perinatal Hepatitis B Prevention Case Coordinator
leovi.madera@state.co.us
Cell 303-870-6263
Fax 303-759-5257